Accelerating Discovery, Optimization, and Delivery
Click chemistry was introduced in 1998 as a conceptual framework for functional molecular assembly, emphasizing the importance of carbon-heteroatom linkages in joining modular building blocks with a few nearly perfect reactions. Click reactions were identified as processes that work under operationally simple, oxygen- and water-tolerant conditions, not influenced by pH, temperature, presence of other functional groups and generate products in high yields with minimal requirements for product purification. The goal was to accelerate the process of discovery, optimization, and delivery, especially those in drug discovery, through practical synthesis.
- Abstr Pap Am Chem S. 1999:ORGA-105, Accession Number 99:145537.
- Angew. Chem. 2001, 40, 2004-2019.
- Angew. Chem. Int. Ed. 2002, 41, 2596-2599.
- J. Org. Chem. 2002, 67, 3057-3064.
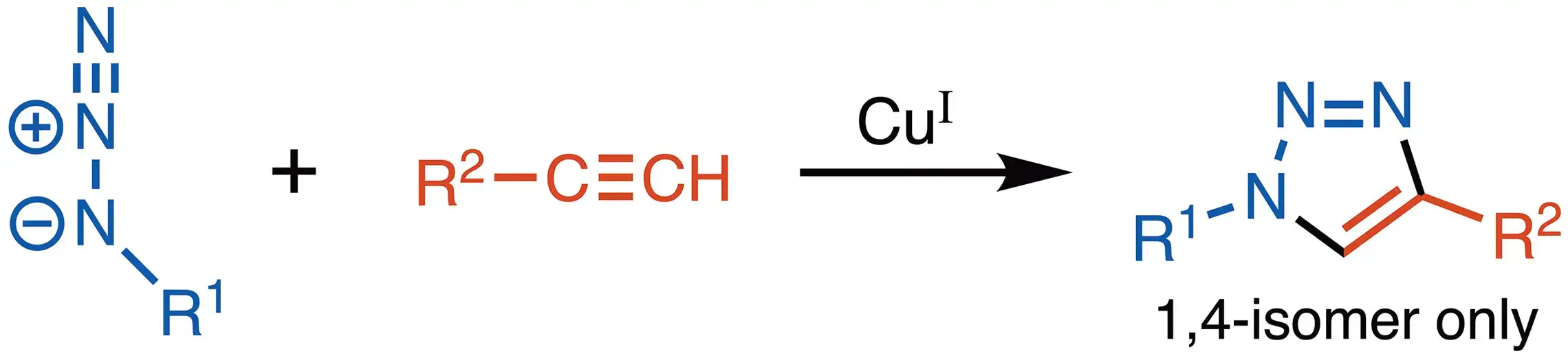
The most powerful click reaction to date is the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). Because azides and alkynes are not found in any of the natural building blocks of life, nor do they react with the most common biomolecules, they are considered to be ‘biorthogonal’. Despite their inertness in a biological milieu, in the presence of a catalytic amount of Cu(I), a terminal alkyne regio-specifically reacts with an azide forming a 1,4-disubstituted triazole.
By exploiting this unique reactivity and selectivity, a number of high-utility techniques have been invented or revolutionized.
Important Applications
Bioconjugation
Regioselective
Site-specific
Bio-orthogonal
Drug Discovery
Modular based on intermolecular linkage builders
In situ click chemistry within a target’s binding sites
“Built-in” scale up capability
Biomedical Imaging
Ligand discovery based on intermolecular linkage builders
In situ click chemistry
Rapid radiolabeling
Materials/Polymer Science
Pure materials due to 99.99+% per-step yields
Easy tuning of material properties (optical, electric)
Click Chemistry II
Sulfur Fluoride Exchange
In 2014, the Sharpless lab discovered Sulfur Fluoride Exchange as a new, nearly perfect family of click chemistry reactions for constructing structurally diverse molecules with unique functions. SuFEx relies on readily available materials and simple transformations to produce compounds bearing the SVI-F motif, such as -O-SO2-F (fluorosulfate) and -SO2-F (sulfonyl fluoride).
S-F incredibly stable
Metal-free conditions
More diverse than CuAAC
New linkages-Molecular “Plugins”
- Angew. Chem. 2014, 53, 9430-9448.
Sulfur(VI) Connectors
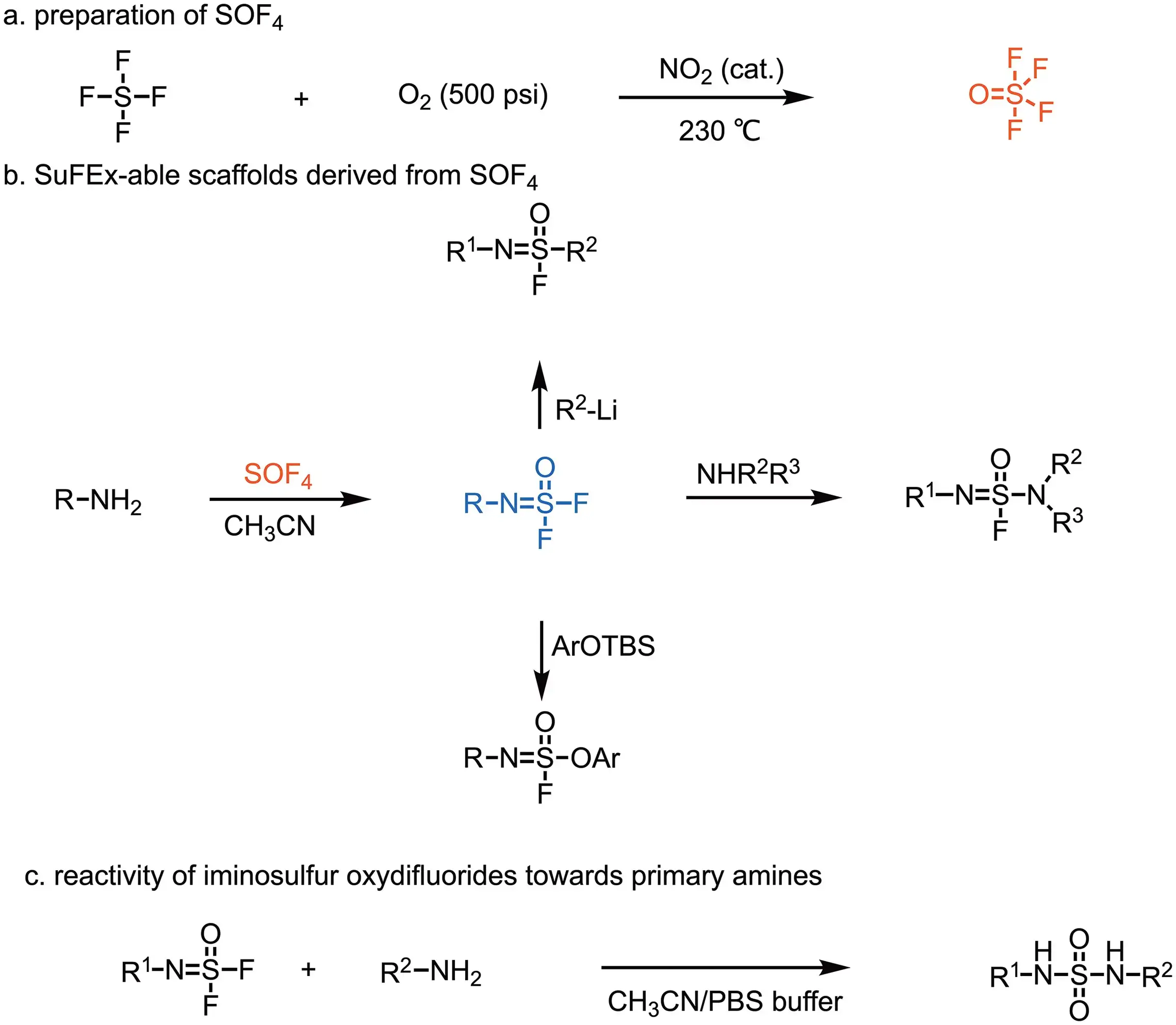
The Sharpless lab established three versatile Sulfur(VI) connectors: sulfuryl fluoride (SO2F2), ethenesulfonyl fluoride (ESF) and thionyl tetrafluoride (SOF4), among which SO2F2 selectively reacts with the -OH group of phenols to form aryl fluorosulfates and SOF4 selectively reacts with amines to generate iminosulfur oxydifluorides.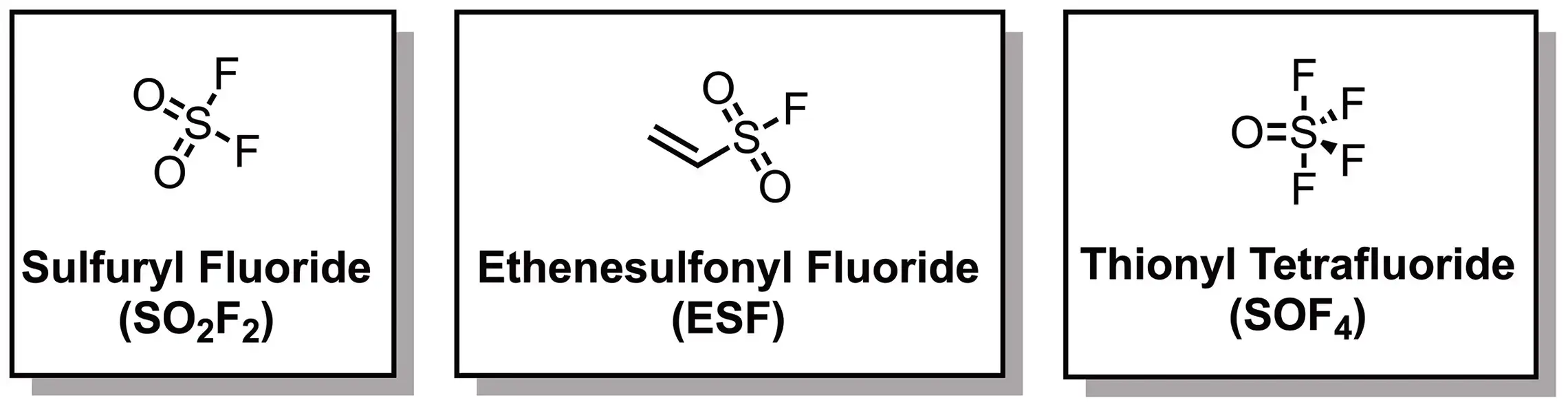
Sulfuryl Fluoride (SO2F2)
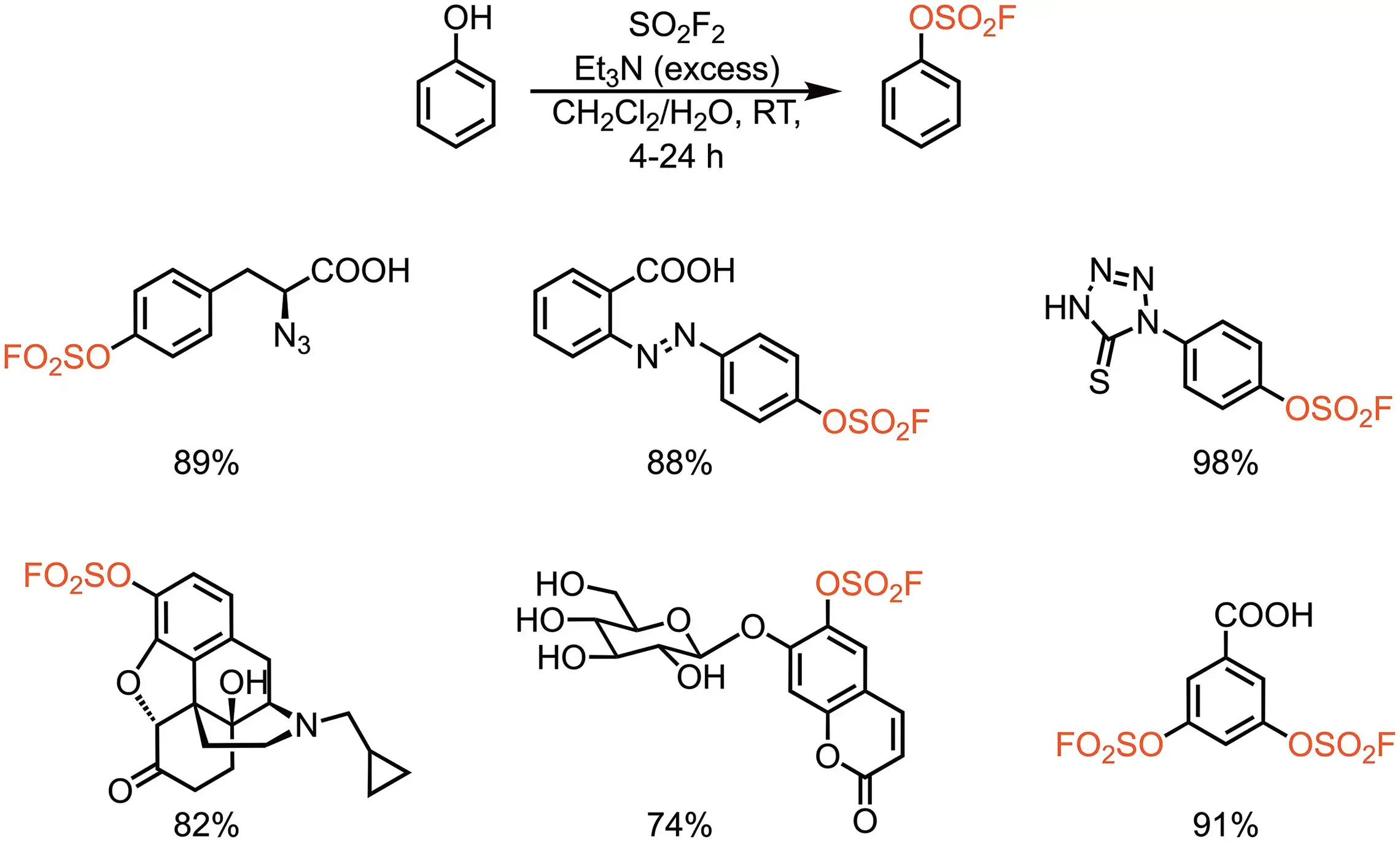
- Angew. Chem. 2014, 53, 9430-9448.
Ethenesulfonyl Fluoride (ESF)
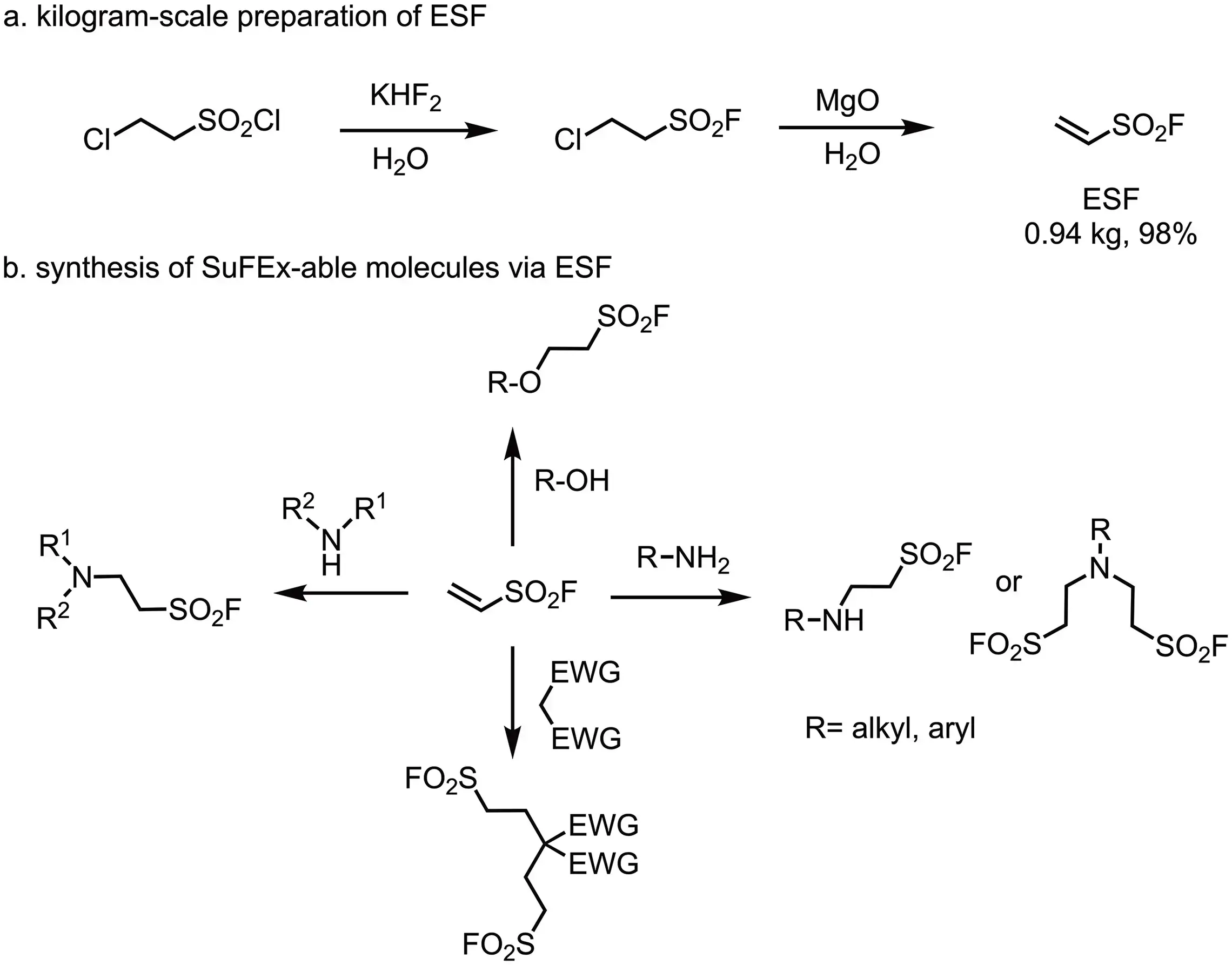
- J. Org. Chem. 2016, 81, 11360−11362
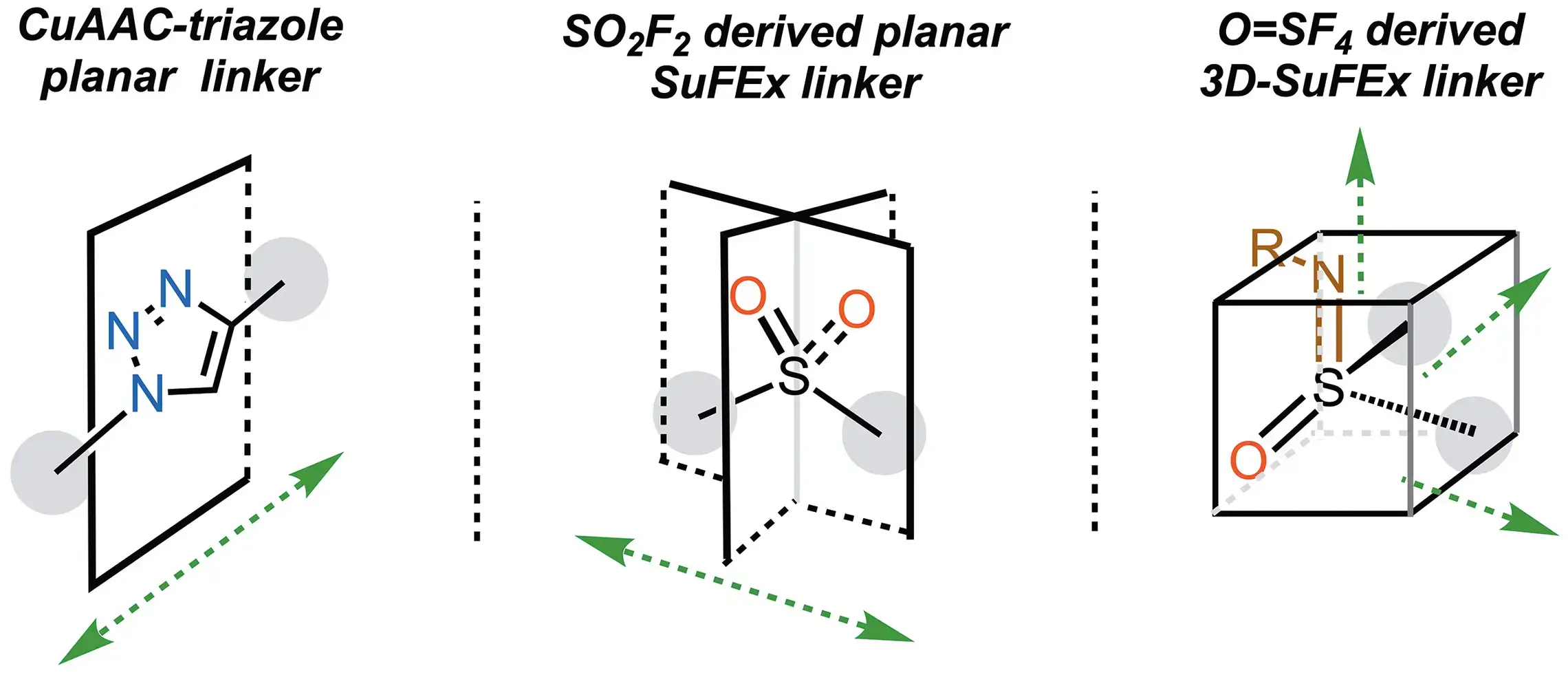
Thionyl tetrafluoride (O=SF4)
Thionyl tetrafluoride is a multivalent electrophile, which allows three different ‘plug-in’ nucleophiles to be sequentially, covalently attached. All three steps are robust, quantitative click reactions.
- Angew. Chem. Int. Ed. 2017, 56, 2903-2908.
- Angew. Chem. Int. Ed. 2018, 57, 1939-1943.
- Angew. Chem. Int. Ed. 2019, 58, 8029-8033.
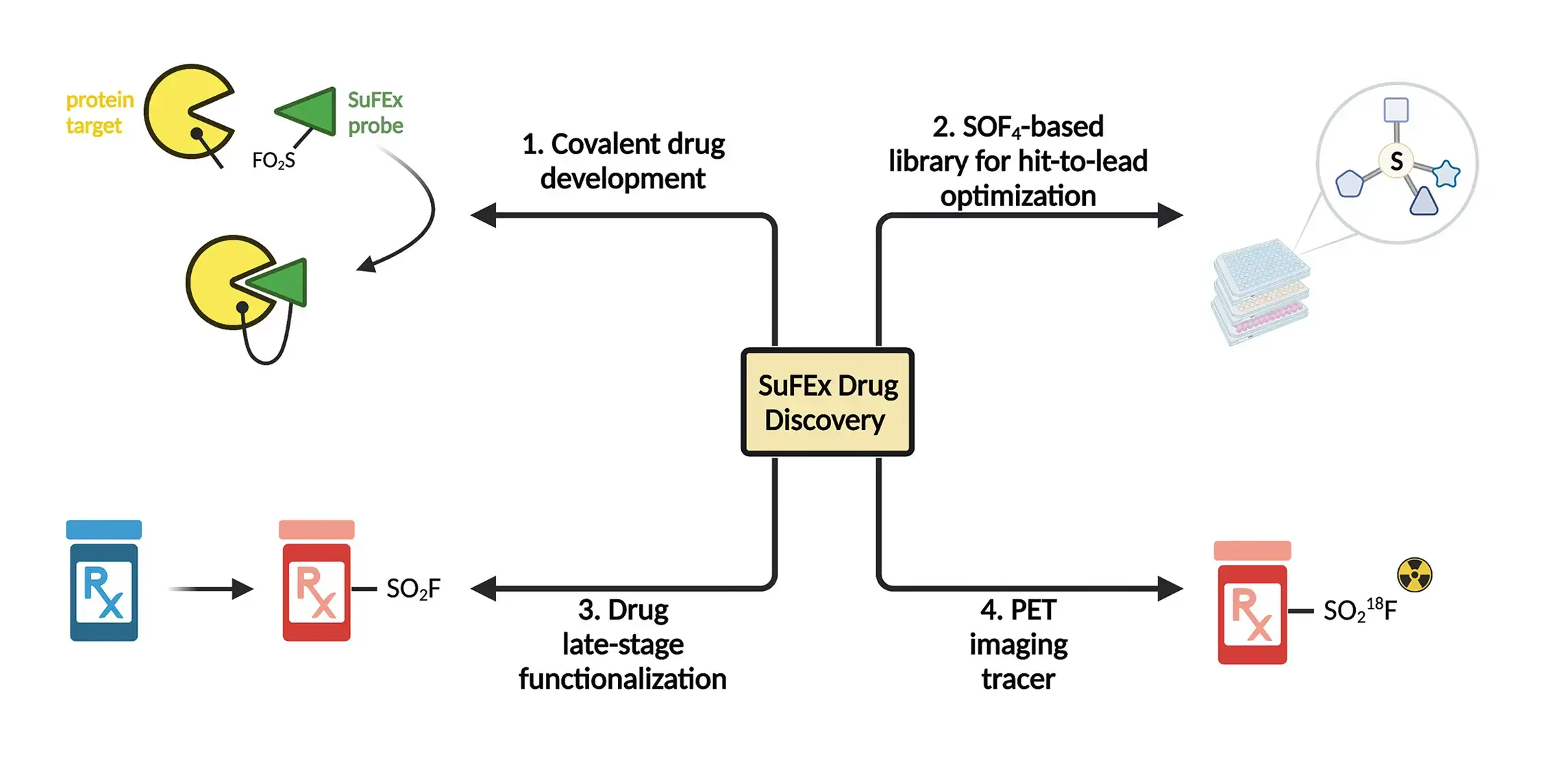
SuFEx-Enabled Drug Discovery
- J. Am. Chem. Soc. 2016, 138, 7353–7364.
J. Am. Chem. Soc. 2017, 140, 200–210.
Nat Chem 2020, 12, 906–913.
Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 18808–18814. - J. Am. Chem. Soc. 2020, 142, 10899–10904.
- J. Am. Chem. Soc. 2018, 140, 2919–2925.
- J. Am. Chem. Soc. 2021, 143, 3753–3763.
—
1. Agnostic screen of SuFEx-able fragment library -serine protease, human neutrophil elastase (hNE)
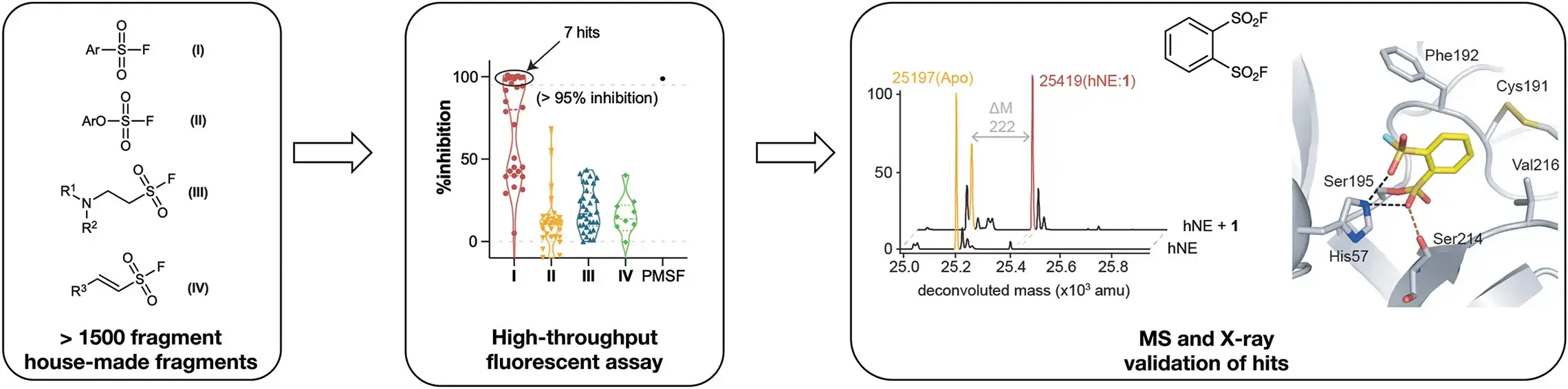
- Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 18808–18814.
—
2. Hit-to-lead optimization via high-throughput SuFEx
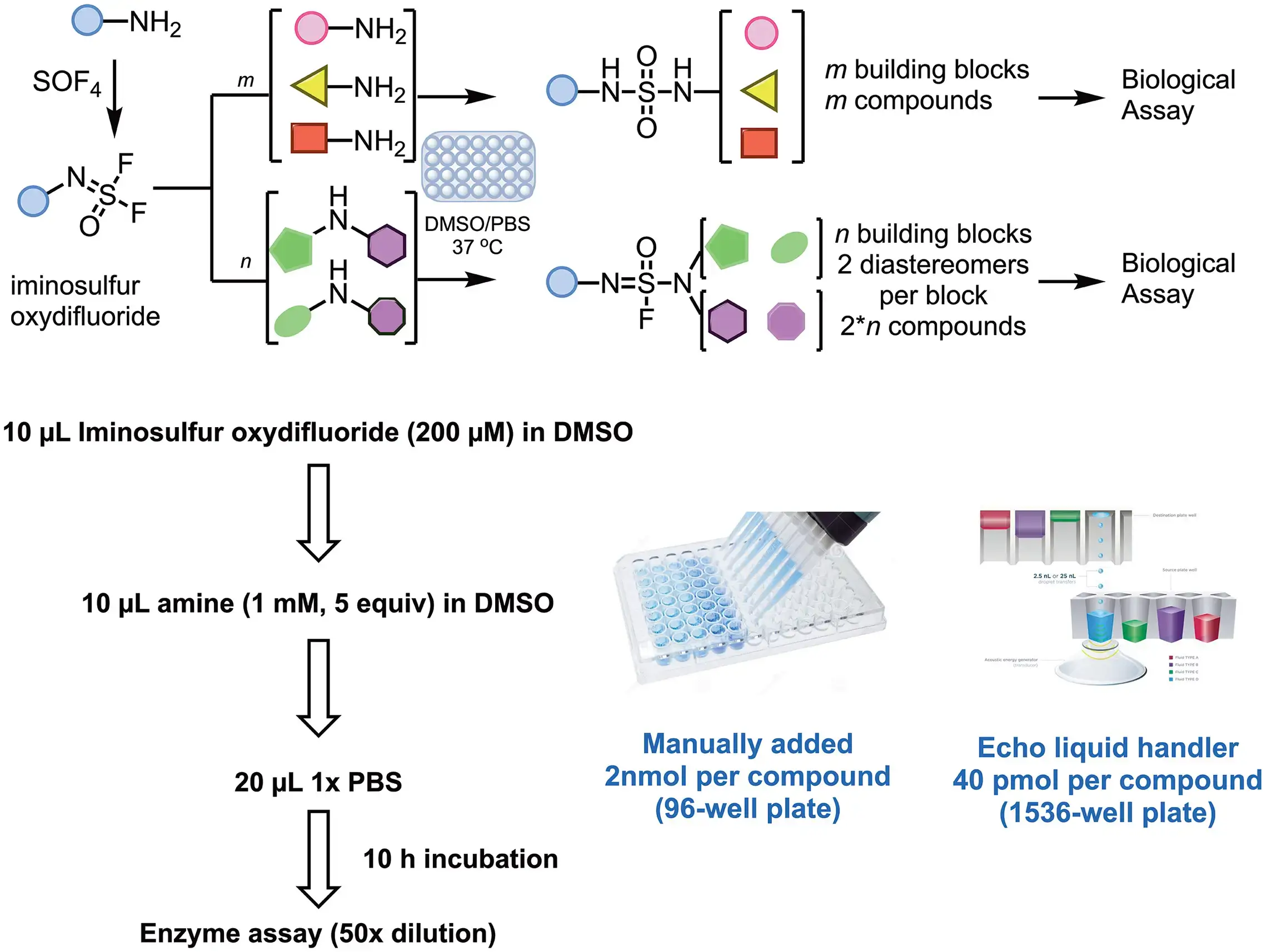
- J. Am. Chem. Soc. 2020, 142, 10899–10904.
—
3. Functionalization and direct phenotypic screening of drug derivatives in microplates
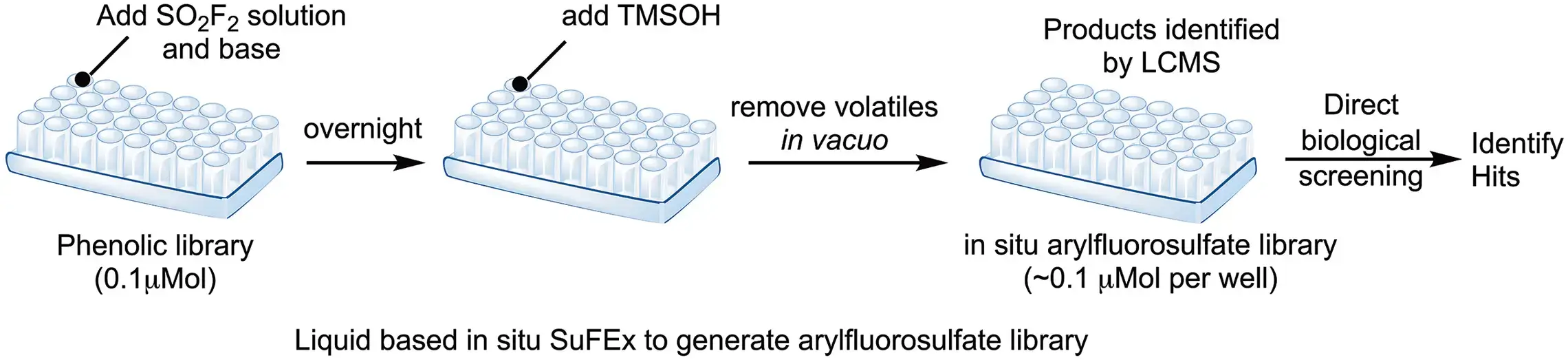
- J. Am. Chem. Soc. 2018, 140, 2919–2925.
—
4. SuFEx-enabled PET tracer development
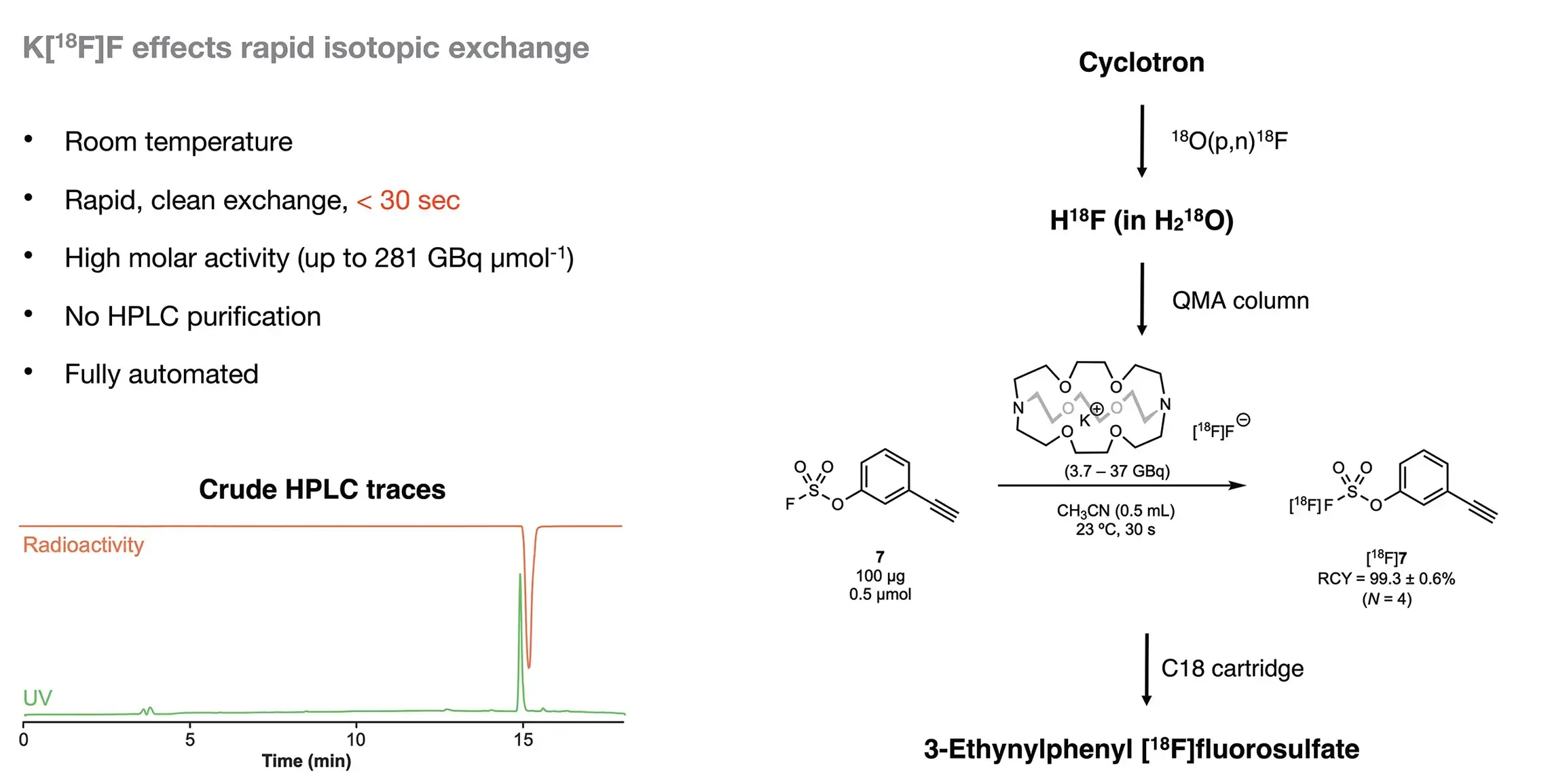
- J. Am. Chem. Soc. 2021, 143, 3753–3763
SuFEx in Materials Science
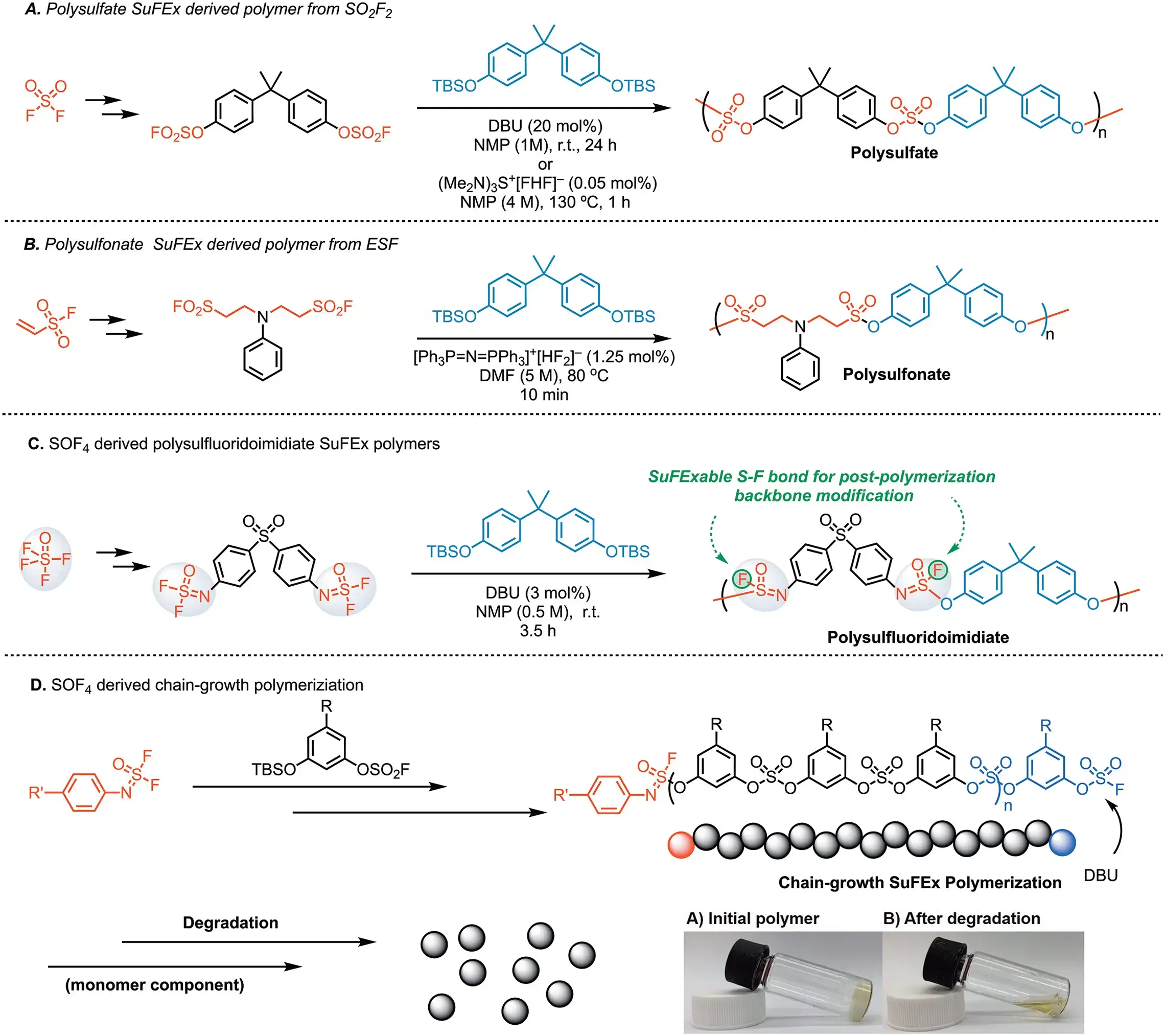
- Angew. Chem. Int. Ed. 2014, 53, 9466–9470.
- Angew. Chem. Int. Ed. 2014, 53, 9430–9448.
Angew. Chem. Int. Ed. 2017, 56, 11203–11208. - Nature Chem. 2021, 13, 858−867.
- ACS Cent. Sci. 2021, 7, 1919−1928.
Polysulfates for High-Temperature, High-Field Capacitive Energy Storage
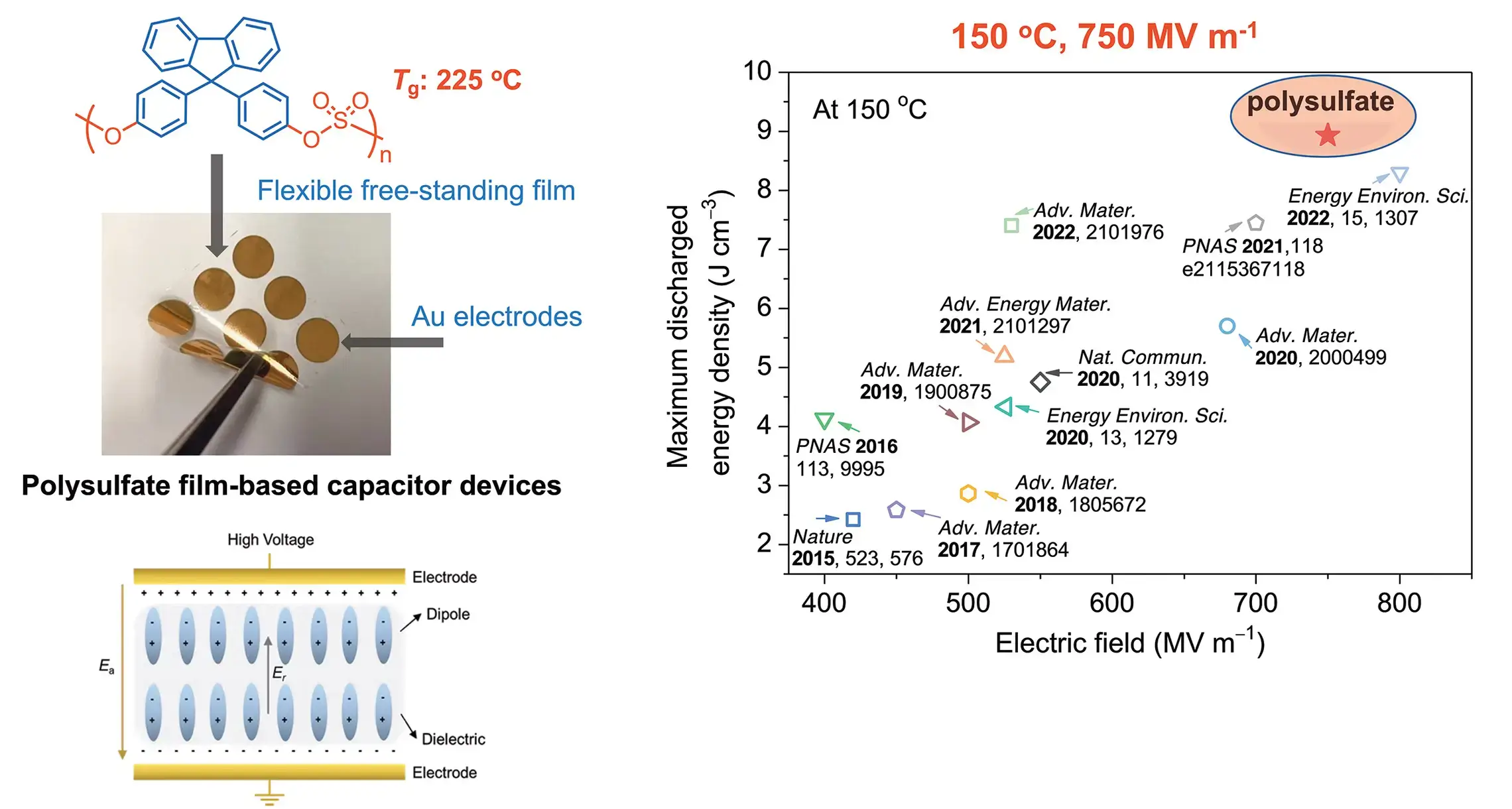
- Joule 2023, 7, 95–111
Collaborators
Jeff Kelly
Professor at TSRI
Covalent drug discovery by proteomics using SuFEx-based covalent probes. (Inverse drug discovery)
Hugh Rosen
Professor at TSRI
Screening of SuFEx library for identifying and evaluating chemical agonists and antagonists of receptor function.
Peng Wu
Professor at TSRI
SuFEx enabled late-stage functionalization and direct phenotypic screening to identify improved drug candidates. Use of SuFEx to develop small molecule ligands for Siglecs. Development of SuFEx based polymers.
Dennis Wolan
Adjunct Professor at TSRI/Genentech
Screening of SuFEx fragment library to discover covalent inhibitors of human neutrophil elastase (hNE). Hit-to-lead optimization via SuFEx-enabled high-throughput medicinal chemistry to develop improved inhibitor of SpeB.
Xiaohua Wu
Professor at TSRI
Use of modular click chemistry to construct SuFEx-derived screening libraries with a focus on identifying small molecule inhibitors towards potential targets that can be used for targeted cancer treatment in checkpoint and DSB repair pathways.
Yi Liu
Facility Director at the Molecular Foundry (Berkeley)
Development of SuFEx based polymer film capacitors to create a new polymer-based device that efficiently handles record amounts of energy while withstanding extreme temperatures and electric fields.
Michael Erb
Professor at TSRI
Using SuFEx for PROTAC development, first-in-class proteolysis targeting chimera (PROTAC) for ENL YEATS domains in acute leukemia.
